1.1. Overview of Module 1
This module provides an overview of vitamin C's general requirements, mechanisms of action, pharmacokinetics (oral and intravenous) and safety. Mechanisms of action specific to certain disease states will be further highlighted in those particular modules.
Following completion of this module the participant will be able to:
- Describe how vitamin C works in the body to colleagues and patients (Communication, Scholarship, Professionalism)
- Explain the difference between oral and intravenous vitamin C to colleagues and patients (Communication, Scholarship, Professionalism)
- Apply knowledge on vitamin C pharmacokinetics to their healthcare practice (Clinical expertise, Professionalism)
- Demonstrate awareness of the potential safety issues with oral and intravenous vitamin C (Clinical expertise, Professionalism)
Length of activity: 40 min (15 min reading + 25 min audiovisual)
RNZCGP Endorsed: 0.65 CME credits
CICM Accredited: 0.65 points
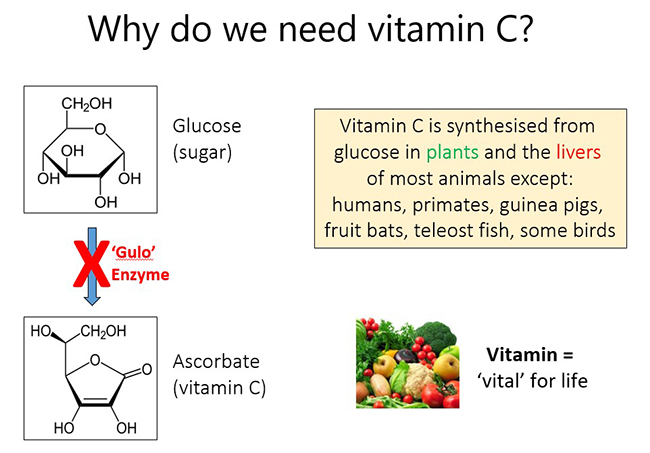
1.2. Why do we need vitamin C?
Vitamin C (ascorbic acid) is a small 'sugar-like' molecule that is required by humans in order to survive. Vitamin C is synthesised by plants and in the livers (or kidneys) of most animals, except humans and a handful of other animal species (e.g. primates, guinea pigs, fruit bats, teleost fish, and some bird species). Humans have lost the ability to synthesise vitamin C from glucose in our livers due to random genetic mutations that have occurred during our evolution. These mutations have resulted in a key enzyme in the biosynthetic pathway (L-gulonolactone oxidase or 'Gulo') being 'knocked out'. As such, vitamin C should really be a normal liver metabolite, but instead it has become a vitamin, meaning it is essential for life and humans must obtain the vitamin through the diet, primarily from fresh fruit and vegetables.
Without adequate dietary intake of the vitamin, hypovitaminosis C and the subclinical signs of deficiency begin to be observed including fatigue, lethargy, and mood changes, e.g. depression. Sustained vitamin C deficiency results in the potentially fatal deficiency disease scurvy, characterized by bruising (petechiae and ecchymosis), bleeding gums, poor wound healing, muscle weakness and bone pain. Although historically observed on long sailing voyages due to inadequate dietary intake of fresh fruit and vegetables, scurvy is still observed today, even in high income countries.

1.3. Vitamin C's role as an enzyme cofactor
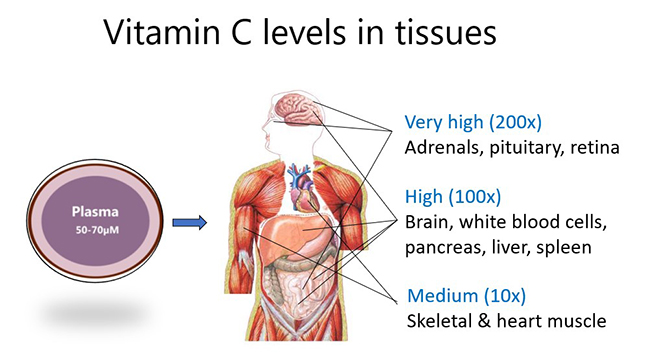
Although plasma levels of the vitamin are usually maintained at ~50-70 µmol/L in healthy people, certain cells, tissues and organs accumulate much higher concentrations of the vitamin. For example, skeletal and heart muscle contain only low concentrations (e.g. 0.2 mmol/L), whereas the brain and particularly the pituitary and adrenal glands contain up to 10 mmol/L vitamin C. This suggests important functions for the vitamin in those tissues.
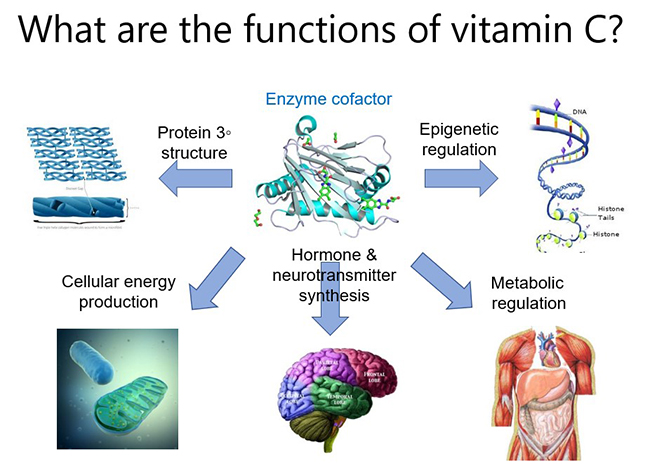
The major function of vitamin C in the body is to act as a cofactor for a family of biosynthetic and regulatory enzymes. These enzymes contain transition metal ions (copper and iron) in their catalytic/active sites and vitamin C is able to donate electrons which regenerates the enzyme active sites. These enzymes are involved in numerous physiological pathways throughout the body, from synthesis of biomolecules such as collagen, carnitine, catecholamines (e.g. noradrenaline), peptide hormones (e.g. vasopressin, oxytocin), to regulation of enzymes involved in epigenetic modifications and gene transcription regulation. Thus, vitamin C can help to up- and down-regulate thousands of genes in the body, which likely contributes to its pleiotropic roles in human health and disease. Note that mechanisms of action specific to certain disease states will be discussed in those particular modules.
1.4. Vitamin C's role as an antioxidant
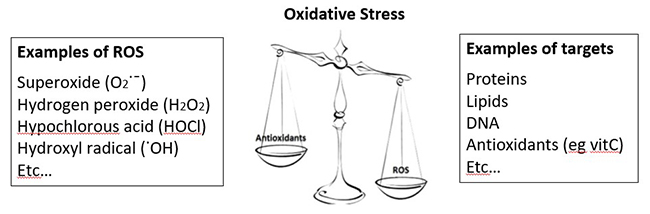
One of the best-known functions of vitamin C is that of antioxidant. Due to its ability to donate electrons, vitamin C is able to help combat 'oxidative stress', which is an imbalance between the amount of reactive oxygen species (ROS) in the body and the ability of the body's antioxidant defense systems to combat these. Reactive oxygen species can be generated endogenously as byproducts of various physiological processes, e.g. mitochondrial oxidative respiration, metabolism of xenobiotics, or generated by white blood cells to help kill pathogens (see examples in figure below). They can also be from exogenous sources e.g. cigarette smoke, air pollution.
Vitamin C is a potent antioxidant which can scavenge a variety of reactive oxygen species, thus protecting important biomolecules such as proteins, DNA and lipids from oxidative damage, thus protecting cells and tissues from damage and dysfunction. The vitamin is also able to regenerate other nutrients (e.g. vitamin E/α-tocopherol) and other enzyme cofactors (e.g. tetrahydrobiopterin) from their oxidised states.
In the process, vitamin C becomes oxidised to the ascorbyl free radical (donation of one electron) or dehydroascorbic acid (donation of two electrons; see figure below). Both of these oxidized species can be readily reduced back to ascorbate (vitamin C) in the body via chemical and enzymatic means, e.g. utilizing glutathione for reducing equivalents. If there are insufficient reducing equivalents, dehydroascorbic acid is rapidly degraded (hydrolysed), resulting in loss of vitamin C from the body.
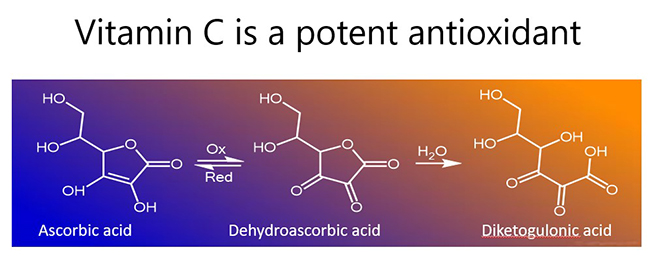
Further reading:
-
Does vitamin C act as a pro-oxidant under physiological conditions? Carr and Frei. FASEB J, 1999.
This review highlights the evidence for vitamin C's antioxidant role in the body, investigated using biomarkers of oxidative stress in different disease states.
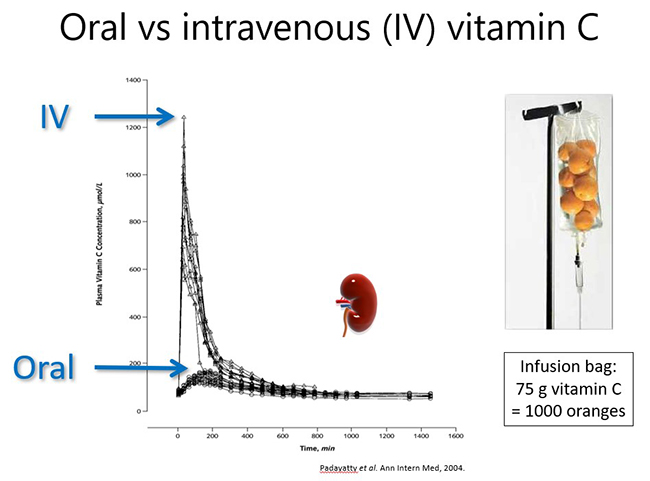
1.5. Pharmacokinetics of oral and intravenous vitamin C
Both oral and intravenous vitamin C have been used for many decades for the prevention and treatment of various diseases. Dietary/oral vitamin C is actively taken up through the small intestine via specialized sodium-dependent vitamin C transporters (SVCT1). These transporters are saturable, i.e. can only uptake a certain amount of vitamin C at any one time (e.g. ~200 mg), therefore, to optimise vitamin C intake, higher doses should ideally be consumed in divided/smaller amounts throughout the day.
Because vitamin C is water-soluble, any excess that is not taken up from the circulation by cells and tissues (via SVCT2) is excreted in the urine (see figure below). Vitamin C can also be reabsorbed within the kidney tubules which helps to maintain whole body levels and conserves the vitamin during times of low intake. Oxidised vitamin C (dehydroascorbic acid) can be transported into cells via glucose transporters (GLUT), where it is reduced back to ascorbate (vitamin C).
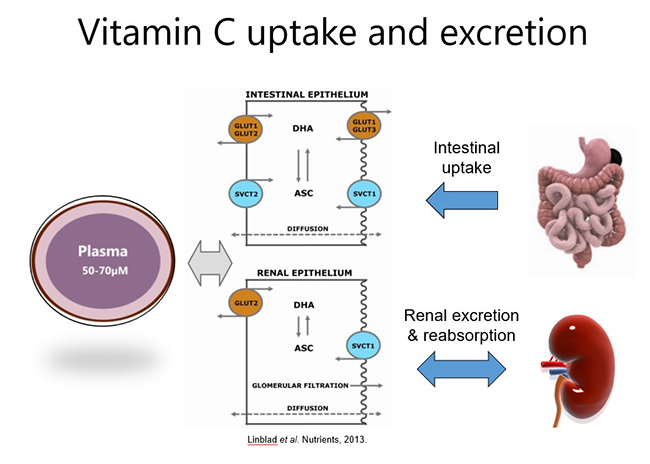
1.5.1. Oral vitamin C pharmacokinetics
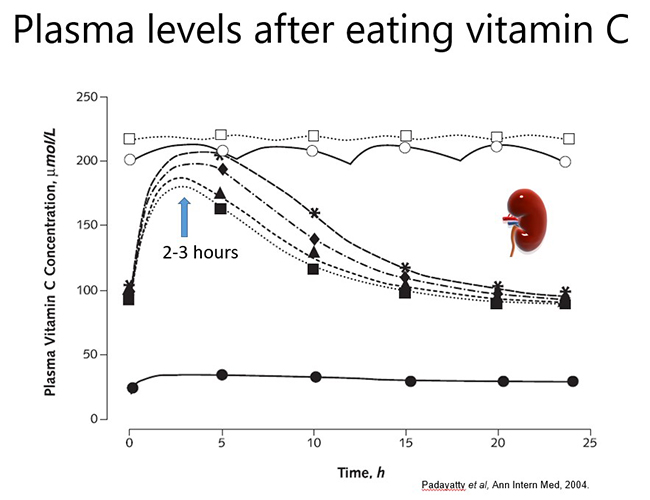
In a healthy person, plasma vitamin C levels peak approximately 2-3 hours after consuming vitamin C, and then return to baseline levels due to renal excretion of excess vitamin (see figure below). Thus, to maintain optimal plasma levels, vitamin C should be consumed regularly throughout the day. However, if a person has inadequate vitamin C status at baseline (e.g. <50 µmol/L), no obvious plasma peak will be observed following consumption of the vitamin because it is readily taken up from the circulation by the depleted tissues.
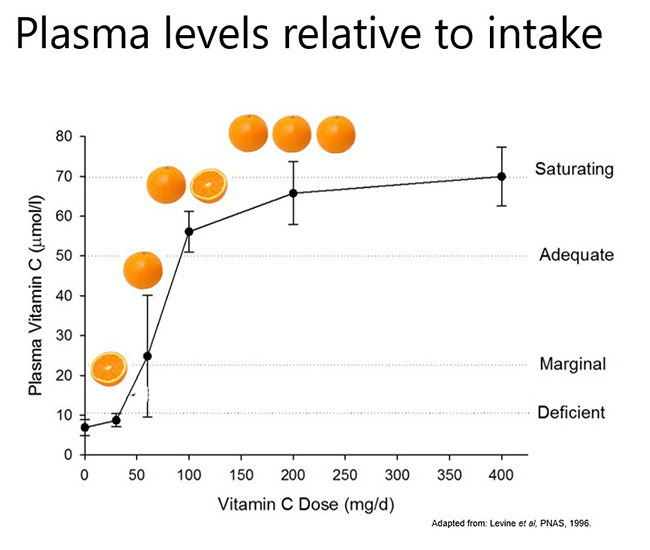
Pharmacokinetic studies have shown that dietary vitamin C uptake is non-linear and instead exhibits sigmoidal kinetics (see figure below). Intakes of ≥200 mg/d result in saturating plasma levels i.e. ~70 µmol/L in healthy people. In people who have an acute illness, such as a severe infection, or an ongoing chronic condition, such as obesity and diabetes, the body's requirement for vitamin C increases and larger doses are needed to reach saturating status. This will be discussed in more detail in the relevant modules.
Plasma concentrations of 50 µmol/L are considered adequate by some authorities (e.g. EFSA – European Food Safety Authority); this concentration can be obtained from ~100 mg/d intake. Plasma concentrations ≤23 µmol/L are when the preclinical signs and symptoms of scurvy can start to appear (e.g. lethargy, fatigue, low mood) and are termed hypovitaminosis C. Concentrations ≤11 µmol/L are considered deficient and at risk of developing scurvy. The overt symptoms of scurvy are generally observed at concentrations <5 µmol/L.
Excretion of vitamin C can be measured in urine samples, however, there is a non-linear correlation between plasma and urinary vitamin C concentrations, with higher concentrations of vitamin C only appearing in the urine once the blood has reached the renal reabsorbtion threshold, resulting in saturating plasma concentrations (i.e. >65 µmol/L). In some conditions, e.g. diabetes, renal leak of vitamin C has been observed in some people even at low plasma concentrations.
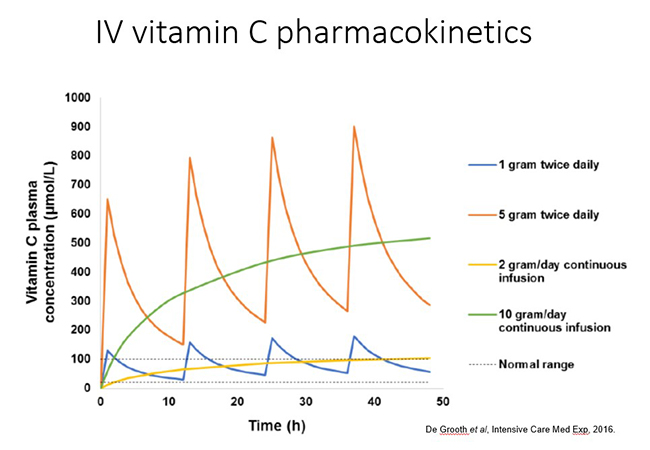
1.5.2. Intravenous vitamin C pharmacokinetics
Intravenous administration of vitamin C is able to bypass the regulated intestinal uptake of the vitamin and results in significantly higher plasma concentrations (see figure and further reading below). Very high doses of vitamin C can be administered this way (which will be discussed in more detail in the relevant modules). However, the resultant high plasma concentrations are still relatively transient due to rapid renal clearance of the vitamin with a half-life in blood of <2 hours.
Different dosing regimens are used depending on the condition being
treated and the mechanisms of action required, i.e. immune/organ
supportive or 'pro-oxidant/pro-drug' role (these will be discussed in
more detail in the respective modules). Thus, oncology patients
generally receive doses of 50-75 g/infusion 2-3x per week. In contrast,
septic patients generally receive 6-15 g/day for 4-7 days (in divided
doses every 6-12 hours; see figure below). Continuous infusions can also
be administered in hospital settings.
1.5.3. Activity: Pharmacokinetic audiovisual
Timing: approx. 25 min
Watch this presentation by Prof Lykkesfeldt on the pharmacokinetics of oral and intravenous vitamin C (from the 'Vitamin C symposium 2019: from bench to bedside').
Further reading:
-
The pharmacokinetics of vitamin C. Lykkesfeldt and Tveden-Nyborg. Nutrients, 2019.
This comprehensive review discusses the complex pharmacokinetics of vitamin C. -
Vitamin C pharmacokinetics: implications for oral and intravenous use. Padayatty et al. Ann Intern Med, 2004.
This study compared oral and intravenous vitamin C pharmacokinetics in healthy volunteers.
1.6 Factors affecting vitamin C pharmacokinetics and requirements
Vitamin C pharmacokinetics and requirements can be affected by demographic and health aspects.
Gender
Epidemiological studies (in high income populations) indicate that females generally have higher vitamin C status than males, despite usually having comparable or lower intakes. Pharmacokinetic studies carried out by Dr Levine and coworkers have also indicated that females achieve slightly higher plasma vitamin C concentrations than males at comparable intakes. This is likely due to their lower body mass. Some countries have higher intake recommendations for men relative to women.
Pregnancy and lactation
When women are pregnant, their vitamin C levels decrease due to haemodilution and increasing weight. Pregnant and lactating women also have higher requirements for vitamin C due to the needs of the developing foetus and growing infant. Most countries have higher intake recommendations for pregnant and lactating women.
Age
The effects of aging on vitamin C pharmacokinetics have not yet been investigated in detail and the epidemiological evidence is inconsistent, with some studies indicating that elderly have lower vitamin C status while other studies (e.g. NHANES) indicate higher vitamin C levels in older people. It is likely that the presence of more comorbidities in elderly people, rather than age itself, is the confounding factor. Children (in high income populations) tend to have higher vitamin C status, and because of their lower weight, have lower requirements than adults.
Smoking
Smoking is known to decrease vitamin C status and increase requirements due to enhanced oxidative stress. Some authorities (e.g. the US IOM - Institute of Medicine) recommend an intake of an additional 35 mg/day of vitamin C to counteract this, although it is believed by some that this amount may be an underestimate.
Obesity
Obesity results in lower vitamin C status at equivalent intakes to lower weight people. This is likely due to a volumetric dilution effect, as well as the presence of elevated inflammation and oxidative stress. This will be covered in more detail in the module on cardiometabolic conditions.
Acute and chronic illnesses
Acute and chronic illnesses can also increase requirements for vitamin C due to enhanced utilisation of the vitamin. The effects of illness on vitamin C pharmacokinetics is particularly apparent with infections, with requirements increasing significantly with the severity of the disease, with septic people having the highest requirements. This will be covered in more detail in the respective modules.
Further reading:
- Discrepancies in global vitamin C recommendations: a review of RDA criteria and underlying health perspectives. Carr and Lykkesfeldt, Nutrients, 2020.
-
Factors affecting vitamin C status and prevalence of deficiency: A global health perspective. Carr and Rowe, Nutrients, 2020.
These two reviews discuss the various factors that affect vitamin C pharmacokinetics and requirements.
1.7. Safety of oral and intravenous vitamin C
There are many myths and misconceptions around the safety of vitamin C. Because vitamin C is water-soluble, any excess that the cells of the body do not take up from the circulation is excreted. As such, there is no known upper level of toxicity. Administration of intravenous vitamin C requires adequate renal function in order to clear the high concentrations from the blood. According to a recent systematic review: “There is no consistent evidence that IV high-dose vitamin C therapy is more harmful than placebo in double-blind randomized controlled trials.” There are, however, some situations that require caution or are contraindications – see below.
1.7.1. Gastrointestinal disturbance
At high oral doses (e.g. ≥4 g/day) some people may experience gastrointestinal discomfort and loose stools due to the osmolality of the unabsorbed vitamin C passing through the large intestine. Consuming divided doses throughout the day would negate this effect. Also, people with specific health conditions, such as severe infections, are believed to be able to tolerate much higher doses due to the enhanced requirements for the vitamin by the body.
1.7.2. Kidney stones/oxalate nephropathy
The belief that vitamin C intake can cause kidney stones has originated from the fact that further degradation of the hydrolysed form of dehydroascorbic acid (i.e. diketogulonic acid) can result in the formation of oxalic acid. Under certain circumstances, excess oxalic acid can crystalise and contribute to the formation of oxalate kidney stones.
Although early research indicated increased oxalate levels in urine following vitamin C supplementation, this was subsequently shown to be primarily due to artefactual ex vivo oxidation of vitamin C in the urine samples. With appropriate handling of urine samples, even very large intravenous doses of vitamin C can be administered with minimal increases of oxalate in urine. Whether increased urinary excretion of oxalate translates to increased renal stone formation is not yet known.
Although a limited number of epidemiological studies have suggested an association between dietary intake of vitamin C and kidney stones, other studies have not shown any link at all. There are, however, many methodological issues with these types of studies. Estimating dietary intake at one point in time and then monitoring incidence of kidney stones at a distant time point is a very inaccurate science. This does not take into account the inherently inaccurate nature of dietary intake estimates (particularly food frequency questionnaires), changes in dietary habits over time, or the variable association between dietary intake and vitamin C levels in the body due to numerous factors that affect body vitamin C status. Furthermore, these types of studies are not able to take into account all the other confounding factors that could contribute, such as dehydration, dietary intake of oxalates and other stone forming compounds, type of stone (e.g. oxalate vs urate, calcium), etc.
There have been a few case reports of people with oxalate nephropathy following vitamin C administration, although this is rare, and due to lack of proper controls it is impossible to know if it was the vitamin C that caused the condition and/or other confounding factors. Nevertheless, people who are predisposed to stone formation should be monitored closely. Furthermore, people with renal dysfunction (and resultant oliguria) should not be given high dose IV vitamin C due to the inability of the kidneys to clear excess vitamin, unless they are receiving renal replacement therapy, as these methods filter out vitamin C.
1.7.3. Haemolysis in G6PD deficiency
Glucose 6-phosphate dehydrogenase (G6PD) deficiency results in the susceptibility of red cells to haemolyse in the presence of enhanced oxidative stress, such as enhanced levels of hydrogen peroxide. High-dose vitamin C is able enter red blood cells and reduce transition metal ions (e.g. iron found in heamoglobin) to a form that can catalyse the generation of reactive oxidants, such as hydrogen peroxide. This cannot be neutralised in people with G6PD deficiency and results in haemolysis and haemolytic anaemia.
G6PD deficiency is more common in certain ethnic groups, e.g. African, Asian, Mediterranean. There have been a few case reports of haemolysis in African men with G6PD deficiency administered high dose IV vitamin C (i.e. 80 g, doses typically administered to oncology patients). Although there has been a more recent case report of haemolytic anaemia in a women administered 30 g of IV vitamin C, there have been no reports of haemolysis in people administered lower dose IV vitamin C (e.g. the divided doses typically used in critically ill patients).
1.7.4. Interference with POC glucometers
High-dose IV vitamin C, being an efficient electron donor, can interfere with point-of-care glucometers that utilise electrochemical detection of blood glucose. This generally produces a falsely high reading (depending on the biochemistry of the monitor) that can result in subsequent excessive administration of insulin. Interference is apparent even after the smaller divided doses of IV vitamin C typically administered to ICU patients if the patient has oliguria as they are unable to clear the infused vitamin C.
Vitamin C does not, however, interfere with the standard laboratory tests for glucose. Therefore, laboratory tests should be used for people receiving high dose IV vitamin C, or point-of-care administered prior to IV vitamin C infusion, or after sufficient hours following infusion to allow time for the blood vitamin C concentrations to decrease to concentrations that don't interfere (typically < 500 µmol/L) (see section on IV vitamin C pharmacokinetics).
1.7.5. Drug interactions
There are no known major vitamin C and drug interactions. There are 11 'moderate' interactions and 16 'minor' interactions (see link below for specifics). Consumption of some drugs (e.g. aspirin) and administration of some chemotherapeutic agents can lower vitamin C status. Conversely, case reports have indicated that consumption of vitamin C may lower the concentration of some drugs or block the activity of some medications (e.g. warfarin).
Additional resources:
Further reading:
-
Harm of IV High-Dose Vitamin C Therapy in Adult Patients: A Scoping Review. Yanase et al. Crit Care Med, 2020.
A systematic review reporting negligible adverse events related to high dose IV vitamin C administration, other than the situations and contraindications mentioned above. -
Vitamin C: intravenous use by complementary and alternative medicine practitioners and adverse effects. Padayatty et al.PLoS One, 2010.
A survey of CAM practitioners which indicated remarkable safety of widely used high dose IV vitamin C therapy.
1.8 Summary of Module 1
- Vitamin C is a water-soluble nutrient that is essential to humans due to loss of our ability to synthesise the vitamin in our livers (as a result of random genetic mutations early in our evolution).
- Vitamin C deficiency can result in the potentially fatal deficiency disease scurvy, which is still observed today, even in high-income countries. Symptoms include malaise, lethargy, mood changes, bleeding gums, skin bruising, poor wound healing, bone pain and muscle weakness.
- Vitamin C acts as a cofactor for a family metalloenzymes with important biosynthetic and gene regulatory functions. The vitamin concentrates in tissues where these reactions take place, e.g. adrenal and pituitary glands.
- Vitamin C is also a potent antioxidant, able to scavenge a wide variety of reactive oxygen species, thus potentially protecting tissues from oxidative damage and dysfunction.
- The vitamin is actively transported into the body and tissues by specialised vitamin C transporters (SVCTs). Plasma 'saturation' occurs when the renal reabsorption threshold has been reached, with excess vitamin C being excreted in the urine.
- Oral vitamin C uptake is restricted by the kinetics of the intestinal vitamin C transporters (SVCT1), therefore higher doses should be taken in divided amounts throughout the day.
- Intravenous vitamin C bypasses the regulated intestinal uptake of oral vitamin C, thus, significantly higher plasma concentrations can be achieved, however, these peaks are relatively transient due to rapid renal clearance of excess vitamin.
- There are a number of factors that affect vitamin C requirements including pregnancy and lactation, gender and age (likely related to differences in body weight), obesity, smoking, and acute and chronic illnesses.
- Oral and intravenous vitamin C are non-toxic and relatively safe, with a few caveats to be aware of: possible gastrointestinal disturbance in some people following large oral doses, possible kidney stones or oxalate nephropathy in some people with renal dysfunction, haemolysis in people with G6PD deficiency who receive high IV doses.
- High plasma concentrations of the vitamin following IV administration can interfere with some point-of-care glucose monitors. There are also some minor to moderate drug interactions to be aware of.
1.9 Activity: Self-reflection and feedback
Once you have completed all the Modules of interest to you, please complete the self-reflection and feedback form online or email to anitra.carr@otago.ac.nz. For members of the RNZCGP, please include your MCNZ number and the modules completed so that your CPD credits can be uploaded to the RNZCGP website. A certificate of completion can be emailed to you if required.
- Self-reflection and feedback form (online)
- Self-reflection and feedback form (DOCX)
© Anitra Carr, 2022. You may not copy, modify, distribute, display, transmit, perform, publish or sell any of the copyrightable material on this website. You may hyperlink to this website but must include the following statement:
"This link leads to the website 'Oral and intravenous vitamin C use in health care' provided by the Nutrition in Medicine Research Group at University of Otago, Christchurch."
